Defective DePuy Pinnacle Hips
 Johnson and Johnson's DePuy subsidiary has manufactured over 100,000 defective hip implant devices that have unfortunately been used in hip replacement surgeries. Our firm represents hundreds of patients dealing with the defective devices and we've filed lawsuits on their behalves to recover monetary damages to compensate them for the pain, discomfort, and mental anguish they're enduring as they've either undergone a revision surgery or are set to have one in the near future. If you have one of these defective devices, we want to speak with you. We may be able to help you recovery the monetary damages owed as a result of Johnson and Johnson's defective device. Call us today to discuss your discomfort and allow us to evaluate your potential legal claims. The consultation is free.
Johnson and Johnson's DePuy subsidiary has manufactured over 100,000 defective hip implant devices that have unfortunately been used in hip replacement surgeries. Our firm represents hundreds of patients dealing with the defective devices and we've filed lawsuits on their behalves to recover monetary damages to compensate them for the pain, discomfort, and mental anguish they're enduring as they've either undergone a revision surgery or are set to have one in the near future. If you have one of these defective devices, we want to speak with you. We may be able to help you recovery the monetary damages owed as a result of Johnson and Johnson's defective device. Call us today to discuss your discomfort and allow us to evaluate your potential legal claims. The consultation is free.
The Doyle Law Firm is now accepting clients who had hip replacement implants manufactured by DePuy Orthopaedics, Inc., identified as the DePuy Pinnacle Metal-On-Metal Acetabluar Cup System installed during hip replacement surgery. DePuy's faulty design in the Pinnacle device has caused patients to undergo a second "revision" surgery to correct the problems. We are evaluating hip replacement complications in the DePuy implant devices and we've begun filing lawsuits nationwide. If you or someone you know has experienced severe pain or other injuries after the implantation of a DePuy Pinnacle® hip replacement device, email attorney Jimmy Doyle or call us at (205) 533-9500 for a free consultation.

DePuy Pinnacle Hip Replacement Attorneys
Doyle Law is reviewing potential hip replacement lawsuits for people that received the DePuy PINNACLE® Modular Hip Replacement System with metal insert/ liner and experienced pain or other symptoms that led to a second hip replacement surgery. The hip replacement devices are manufactured by DePuy Orthopaedics, a division of Johnson & Johnson Services, Inc., and the total hip replacement system include the cup portion of a replacement hip.
DePuy Pinnacle Lawsuits
The United States Judicial Panel on Multidistrict Litigation chose to centralize all products liability cases involving the Pinnacle hip devices in U.S. District Court for the Northern District of Texas.
There are now hundreds of Pinnacle lawsuits filed and more are sure to come. An MDL benefits all parties involved through the open sharing of pretrial disclosures, the elimination of inconstant pretrial rulings, and a more streamlined litigation process. MDL status differs from class action status because MDL plaintiffs maintain individual lawsuits and trials, which increases their chances of obtaining an amount of compensation that corresponds directly with their damages.
Ongoing Concerns with DePuy Pinnacle Implants
Metal-on-metal devices were developed to add durability as an alternative to the typical plastic device. Latest reports claim that over 1,300 complaints have been received by the FDA concerning the DePuy Pinnacle hip replacement. Even though a significant amount of these complaints focus on the device loosening prematurely, additional news from the New York times claims that such metal-on-metal devices, like the Pinnacle system, increase the risk of metal toxicity causing a number of adverse affects in recipients.
In January, DePuy announced that it had set aside nearly a billion dollars for ASR lawsuits. This amount will surely increase should the Pinnacle lawsuits be added to existing DePuy MDL. If you feel your quality of life has been affected by a defective artificial hip replacement device, do not hesitate to email attorney Jimmy Doyle or call us at (205) 533-9500 for a free consultation.
Metal-On-Metal Artificial Hip Problems
Problems linked to the DePuy Pinnacle Artificial Hip Implants include:
- Blood test results that show high levels of chromium and cobalt caused by metal debris
- Clicking, popping or grinding in the area of the hip implant
- Device failure from loosening that results from bone loss
- Dislocation (the two parts of the implant move against one another and become misaligned)
- Fracture (bone around the implant breaking)
- Loosening in the hip joint (implant fails to stay attached to the bone in the correct position)
- Metal debris (component parts move together resulting in flecks of metal shavings that spread around the area surrounding the implant and destroy soft bone and tissue)
- Problems involving walking, pain, swelling or discomfort in the hip area
- Any of the above symptoms or related conditions that result in revision surgery to remove a Depuy hip device
If you have experienced serious hip replacement side effects or required additional surgery after being implanted with a DePuy Pinnacle Artificial Hip Replacement System, you may have grounds for a hip replacement lawsuit.
Contact an Alabama Hip Replacement Lawyer
If you or someone you know has experienced pain or required revision surgery following the implantation of the DePuy PINNACLE Hip Replacement System, please contact Alabama hip replacement lawyer Jimmy Doyle by calling (205) 533-9500 to discuss your potential hip replacement lawsuit. There's no obligation and the initial consultation is free.

Results Matter
As a result of our preparation and diligent efforts, the lawyers on our team have been able to recover over sixty-three million dollars ($63,000,000.00) for the clients we represent. Those cases have involved dangerous drugs, truck and auto accidents, medical malpractice, construction accidents, fraud, product liability, electrocutions, nursing home abuse, and wrongful death.
DePuy Pinnacle Lawsuit Statute of Limitations
Alabama has a two year statute of limitations on filing certain product liability and personal injury claims. It takes time to acquire medical records and draft pleadings, so please do not delay in contacting us if you have any concern that your metal-on-metal hip replacement device may be defective.

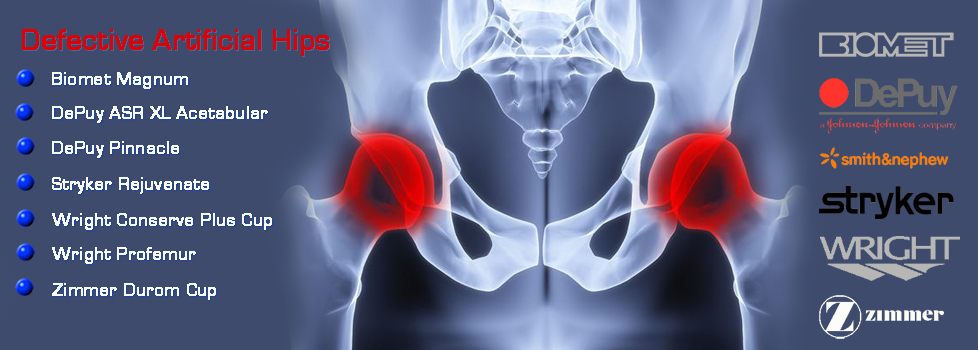


 Fosamax, Boniva, Actonel, Aredia, and Zometa - medication prescribed to treat osteoporosis - are linked to femur fractures after 3 years of use. If you took any of this medication (or any combination thereof) and suffered a femur fracture,
Fosamax, Boniva, Actonel, Aredia, and Zometa - medication prescribed to treat osteoporosis - are linked to femur fractures after 3 years of use. If you took any of this medication (or any combination thereof) and suffered a femur fracture,  Mesh used during surgery to repair the vaginal wall has caused women to experience mesh erosion into the vagina, prolapse, pain during intercourse, incontinence, bowel or bladder perforation, bleeding and serious infection. We're now accepting clients with claims against AMS, Bard, Boston Scientific, Ethicon, and Johnson & Johnson. If you took have suffered any of these side effects,
Mesh used during surgery to repair the vaginal wall has caused women to experience mesh erosion into the vagina, prolapse, pain during intercourse, incontinence, bowel or bladder perforation, bleeding and serious infection. We're now accepting clients with claims against AMS, Bard, Boston Scientific, Ethicon, and Johnson & Johnson. If you took have suffered any of these side effects,  A growing number of metal-on-metal artificial hip implants are being recalled due to early failures and some patients suffering from metallosis - metal debris released from the hip compontents inflame the surrounding tissue and enter the bloodstream, causing elevated levels of cobalt and chromium. We're now accepting clients with claims aginst Biomet, DePuy, Stryker, Smith & Nephew, Wright, and Zimmer. If you have (or had) one of these devices, we can help to protect your legal rights. To contact us,
A growing number of metal-on-metal artificial hip implants are being recalled due to early failures and some patients suffering from metallosis - metal debris released from the hip compontents inflame the surrounding tissue and enter the bloodstream, causing elevated levels of cobalt and chromium. We're now accepting clients with claims aginst Biomet, DePuy, Stryker, Smith & Nephew, Wright, and Zimmer. If you have (or had) one of these devices, we can help to protect your legal rights. To contact us, 